find the number of neutrons si|How to Find the Number of Neutrons in an Atom: 11 Steps : Baguio An atom is the smallest particle of an element that has no independent existence but is directly involved in chemical reactions as the smallest unit. Atoms are . Tingnan ang higit pa We would like to show you a description here but the site won’t allow us.
PH0 · Silicon
PH1 · Protons, Neutrons, Electrons for Silicon (Si, Si4+, Si4
PH2 · How to find the Number of Protons, Electrons, Neutrons for
PH3 · How to Find the Number of Protons, Neutrons, and
PH4 · How to Find the Number of Neutrons in an Atom: 11 Steps
PH5 · Find the Number of Neutrons Si
PH6 · 4.8: Isotopes
PH7 · 2.4: Neutrons: Isotopes and Mass Number Calculations
answer: ang masamang epekto ng online games ay ang pagkalabo ng mga mata at adiksyon nito.. explanation: hope that helps. #carry on learning シ︎
find the number of neutrons si*******Based on the atomic number, mass number, and neutron number of the element, three things can be considered. These are isotope, isobar, and isotone. The number of neutrons depends on the isotope of the atom. Video for Number of Protons, Electrons, Neutrons for Silicon (Si) Tingnan ang higit pa
An atom is the smallest particle of an element that has no independent existence but is directly involved in chemical reactions as the smallest unit. Atoms are . Tingnan ang higit pa
Atoms that have the same number of protons but different mass numbers are called isotopes of each other. The English chemist . Tingnan ang higit pa
An atomic number is a number that carries the properties of an element. The number of electrons and protons in an element is determined . Tingnan ang higit paWhen an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. The ionic properties of the elements depend on the exchange . Tingnan ang higit pa Since the vast majority of an atom’s mass is found its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic . 145. 16K views 3 years ago. In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Silicon (Si). From the Periodic.
The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic .To find the number of protons in silicon, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since silicon 's .
Number of neutrons contained in the atom; Solutions. The atomic number of an element is found above the elemental symbol within a box on the periodic table. The element with an atomic number of 74 is symbolized .The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, .
find the number of neutrons siThe total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A . The .How to Find the Number of Neutrons in an Atom: 11 Steps Part 1. Calculating Protons, Electrons, and Neutrons. Download Article. 1. Get a periodic table of elements. The periodic table .Introductory Chemistry. 4: Atoms and Elements. 4.8: Isotopes - When the Number of Neutrons Varies. Expand/collapse global location. 4.8: Isotopes - When the Number of .The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons. CAS number
find the number of neutrons si How to Find the Number of Neutrons in an Atom: 11 Steps The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons. CAS number The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number of Neutrons in Silicon = Atomic mass of Silicon – Atomic number of Silicon = 28 – 14 = 14. Number of Electrons in Silicon. For a neutral atom, the number of electrons can be found by knowing the atomic number of that atom.
(ii) neutron (iii) electron (iv) nucleon (d) If the atomic number of an atom is 17 and mass number is 35 then number of neutrons will be (i) 35 (ii) 17 (iii) 18 (iv) 52 (e) The number of electrons in an atom is equal to the number of (i) protons in a neutral atom (ii) neutrons in a neutral atom (iii) nucleons in a neutral atom (iv) none of the .
The sum of the numbers of protons and neutrons in the nucleus is called the mass number and, expressed in amu, is approximately equal to the mass of the atom. An atom is neutral when it contains equal numbers of electrons and protons. . Read more about the redefinition of SI units including the kilogram here (Laura Howe, CE&N, Nov. 16, 2018 .
The number is the atomic mass. Subtract the atomic number of the element from the atomic mass of the isotope, and the result is the number of neutrons in the nucleus of that isotope. In the case of C-14, the atomic number of carbon is 6, so there must be 8 neutrons in the nucleus. That's two more than the more common, balanced .
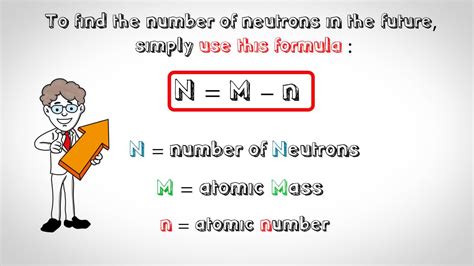
Lithium has 3 protons, 4 neutrons and 3 electrons. 4. Beryllium has 4 protons, 5 neutrons and 4 electrons. 5. Boron has 5 protons, 6 neutrons and 5 electrons. 6. Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element. # of protons = atomic number # of neutrons = mass number – atomic number # of electrons = atomic number – charge. That’s it! Examples. Great, lets apply the rules to some examples. # of protons = 17 # of neutrons = 37 – 17 = 20 # of electrons = 17 – 0 = 17 # of protons = 16 (the atomic number is not given, but can be found on the .
To find the number of protons in silicon, first locate the element on the periodic table. Next, find the atomic number which is located above the element 's symbol. Since silicon 's atomic number is 14, Si has 14 protons. 14 protons. Fill in the known values where N represents the number of neutrons. 28 = 14+N. Rewrite the equation as 14+N = 28.
Number of neutrons = mass number – atomic number. Key fact. Remember that Protons are Positive, and Neutrons are Neutral. Question. The atomic number of a sodium atom is 11 and its mass number .
The charge of an atom is defined as follows: Atomic charge = number of protons − number of electrons (1.8.1) (1.8.1) Atomic charge = number of protons − number of electrons. As will be discussed in more detail later .
An element with three stable isotopes has 82 protons. The separate isotopes contain 124, 125, and 126 neutrons. Identify the element and write symbols for the isotopes. Given: number of protons .
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but . Using the average atomic mass, calculate the average number of neutrons by first rounding the atomic mass from 200.592 to 201. Now, subtract the number of protons, 80, from the atomic mass, 201-80, to find the average number of neutrons, 121. If the mass number of an isotope is known, the actual number of neutrons can be .
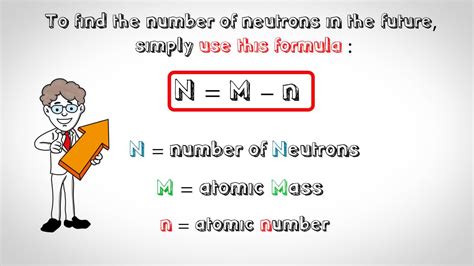
Figure 3.4.1 3.4. 1: The social security number subatomic-the proton. Since atoms are neutral, the number of electrons in an atom is equal to the number of protons. Hydrogen atoms all have one electron occupying the space outside of the nucleus. Helium, with two protons, will have two electrons.The Mass number is equal to the number of Protons plus the number of Neutrons in an atom, so by subtracting the Atomic number from the Mass number we can determine that there are 28 - 14 = 14 Neutrons in Silicon. In an uncharged atom, the number of Electrons equals the number of Protons, so there are 14 Electrons in an atom of Silicon. Using a .The atomic mass, represented here as '29' in Si-29, is the total number of protons and neutrons. Thus, to find the number of neutrons, you subtract the number of protons from the atomic mass. In this case, we subtract the 14 protons from the atomic mass of 29 (29 - 14) to get the number of neutrons. Hence, an atom of Si-29 contains 15 neutrons.
Finding the Number of Neutrons. The number of neutrons in an atom can be calculated by subtracting the atomic number from the atomic mass. Both of these numbers can be found on the periodic table. The atomic number is listed above the symbol of the element whereas the mass number is placed below. Let’s keep using oxygen as our example. Its .
En 4 Fotos 1 Palabra, cada nivel te da 4 fotos y espacio para 1 palabra. Tiene que estudiar las fotos y adivinar la respuesta correcto para continuar a otros niveles. En cada nivel hay letras que tiene que usar para introducir la respuesta. También, si tiene dificultad, hay tipos diferentes de consejos que puede usar para ayudar. 4 Fotos 1 .
find the number of neutrons si|How to Find the Number of Neutrons in an Atom: 11 Steps